Abstract
Introduction: Diffuse large B-cell lymphoma (DLBCL) represents a biologically and clinically heterogeneous diagnostic category with well-defined cell-of-origin (COO) subtypes, each arising from different stages of normal B-cell development. While the prognostic impact of the distinct COO subtypes has been confirmed in several studies, including the GOYA trial (NCT01287741; Vitolo et al. J Clin Oncol 2017), the impact of the key genomic alterations and their dependence on COO are less clear. Using data from the GOYA study, the largest prospective randomized trial in previously untreated DLBCL, we characterized the mutational profile of DLBCL by targeted next-generation sequencing (NGS), and evaluated the prognostic impact of somatic mutations and its relation to COO.
Methods: Diagnostic formalin fixed paraffin-embedded tissue biopsies collected from patients (pts) with previously untreated DLBCL were tested at central laboratories. COO was assessed using the NanoString Research Use Only Lymphoma Subtyping (LST) gene expression assay (NanoString Technologies, Inc., Seattle, WA, USA). COO was evaluable in 933 pts (66% of the GOYA intent-to-treat [ITT] population of 1418 pts). Targeted DNA NGS was performed using the Foundation One Heme™ platform (all coding exons of 465 genes, select rearrangements in 31 genes) in 499 pts (35% of the ITT population). NGS and COO were available for 482 pts (34% of the ITT population). Only genetic alterations/mutations (single nucleotide variants [SNVs], copy number abnormalities [CNAs], chromosomal translocations) with known somatic and functional status were included in the statistical analysis, which was conducted as previously described (Frampton et al. Nat Biotechnol 2013). Multivariate Cox-regression was used to evaluate the prognostic effect of gene alterations with multiple testing adjustment using the Benjamini-Hochberg false-discovery rate (FDR) procedure ("significance" <5% FDR).
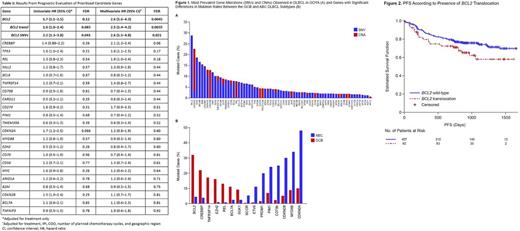
Results: Baseline patient and disease characteristics were similar between pts with COO and/or NGS available, and the overall ITT population, except for geographic region and race. According to COO, 272/482 (56.4%) pts had germinal center B-cell-like (GCB), 78/482 (16.2%) unclassified, and 132/482 (27.4%) activated B-cell-like (ABC) DLBCL, which was similar to the overall COO distribution in GOYA. Of the 465 genes analyzed, we identified 59 genes with mutations (13%) occurring in at least 10/499 pts (≥2% prevalence), with an additional 334/465 genes with mutations occurring in at least 1 pt. The median number of gene alterations per pt was 6 (range 0-17). Overall, 3% of pts had no identified mutation and 93% of pts had multiple alterations (≥2 gene mutations). SNVs were the most common mutation type, while CNAs were specific to a few genes, including CDKN2A/B and REL (Figure 1A). Genes with significant differences in mutation rate (<5% FDR) between the GCB and ABC subtypes are shown in Figure 1B.
On multivariate analysis, only alterations in BCL2 were associated with shorter progression-free survival (PFS) independent of COO, International Prognostic Index (IPI), treatment arm and number of planned chemotherapy cycles (Table 1). This effect was observed for both BCL2 translocations and BCL2 SNVs (Table 1; Figure 2), although the vast majority of pts with BCL2 SNVs also harbored BCL2 translocations (n=29/39; 74%), possibly explaining the negative prognostic impact of BCL2 SNVs. BCL2 alterations were detected in 102/499 pts, with 92/102 having BCL2 translocations, 90% (83/92) of which had GCB subtype DLBCL. Of the 39 pts with BCL2 SNVs, 80% (31/39) had GCB subtype and 15% (6/39) had ABC subtype DLBCL. BCL2 alterations were also significantly correlated with BCL2 gene and protein expression levels.
Conclusions: Our data confirm the molecular heterogeneity of DLBCL, with potential treatment targets harbored by the distinct COO subtypes. Only alterations in BCL2 were associated with clinical outcome independently of of other factors (COO, IPI).
Bolen: Genentech: Employment, Equity Ownership. Klanova: F. Hoffmann-La Roche Ltd: Employment, Other: GALLIUM and GOYA are sponsored by F. Hoffmann-La Roche Ltd. Third-party editorial support, under the direction of Magdalena Klanova, was provided by Lynda McEvoy of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd. Trněný: BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Sehn: Amgen: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Vitolo: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; Gilead: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria. Fingerle-Rowson: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Nielsen: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Lenz: Janssen: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria. Oestergaard: F. Hoffmann-La Roche Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal